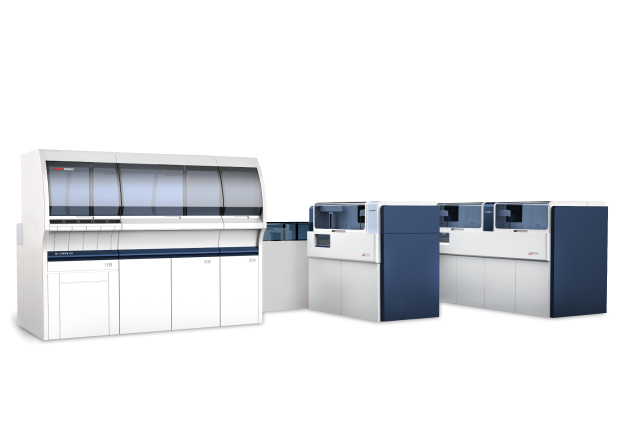
On 7 June, 2025, the Chinese Medical Doctor Association's Physician-Laboratorian Annual Conference & the 19th National Conference on Laboratory Medicine and Clinical Practice came to a close at the Suzhou International Expo Center. With the theme "Better Serving People's Health through Laboratory Medicine" the conference brought together experts from across the country to discuss core topics such as innovation in laboratory medicine, smart lab construction, and improved testing capabilities. Shanghai Sun Biotech Co., Ltd. showcased its UL-2000LAS Fully Automated Coagulation Testing Line and UP/UG/UR Series Coagulation Analyzers, demonstrating its technological strength in advancing standardized testing and supporting the national tiered diagnosis and treatment policy.

Intelligent Coagulation Testing Line: Elevating Diagnostic Efficacy
Amidst China's push for tight-knit medical consortia and mutual recognition of test results, laboratories demand high efficiency, precision, and intelligent solutions. Sun Bio's self-developed UL-2000LAS system features: Dual Emergency Management, prioritizes critical samples with flexible queue-jumping mechanisms.
Stable Sample Transfer, dual-layer tracks with polymer-based silent rails ensure reliability.
RFID Tube Tracking, intelligently allocates samples to analyzers.
On-track Cap-piercing Technology, prevents sample stagnation and boosting turnaround efficiency.
AI Vision and HIL Monitoring, integrated multi-wavelength anti-interference technology guarantees result accuracy. The UL-2000LAS front-end module occupies only 1.99 m²and connects to UR-Series analyzers. The UR8000 Coagulation Analyzer achieves speeds of 900 tests per hour, providing a high-efficient solution for clinical labs.

Integrated Optical and Mechanical Coagulation Detection
Aligned with China's "14th Five-Year Plan for Medical Equipment Development", Sun Bio's UG2500 Analyzer integrates: Optical, Mechanical, and Platelet Aggregation Detection: Unifies coagulation and platelet function analysis.
Smart Mode Switching: Mitigates interference from hemolyzed, lipemic, or icteric samples. This design meets national strategic demands for high-precision, multifunctional devices.

Strict Quality Control: Enabling Test Result Mutual Recognition
Per the "Guidelines on Further Promoting Mutual Recognition of Medical Test Results":
By the end of 2025, tight-knit medical alliances must achieve full mutual recognition of all test items.
Municipal-level institutions must recognize over 200 items.
Quality assurance system ensures standardization. Sun Bio has achieved nearly full scores for 17 consecutive years in the External Quality Assessment (EQA) program administered by the National Health Commission. Additionally, Sun Bio has also participated in the establishment of national industry standards for coagulation diagnostics, including PT, APTT, TT, FIB, D-Dimer, FDP, AT-III, and anti-Xa. The company has promoted the standardization of testing processes, providing technological assurance for the mutual recognition of results among medical institutions.
SunBio remains committed to technological breakthroughs and quality enhancement in the field of coagulation diagnostics. At the exhibition, the UL-2000LAS automated testing line and UP, UG, and UR Series Coagulation Analyzers garnered high recognition from industry experts for their high efficiency, precision, and intelligence.

About Shanghai Sun Biological Technology Co., Ltd.
Founded in 2001, Sun Bio specializes in thrombosis and hemostasis diagnostics, providing global solutions for reagents, instruments, and consumables.
The company strictly adheres to the quality management system and has passed dual international quality system certification to ISO9001 and ISO13485 standards. Our core products have independent intellectual property rights, with more than 100 patents. Meanwhile, we have actively participated in the formulation of national industry standards for PT, APTT, TT, FIB, D-Dimer, anti-Xa and coagulation analyzer, and promoted the standardization of coagulation testing technology. The company has been awarded many honors and qualifications, such as Ministry of Industry and Information Technology’s "Specialized, Refined, Unique, and New" Enterprise, National High-Tech Enterprise, Shanghai Tech Pioneer so on.
In the future, SunBio will deepen R&D in Thrombosis and Hemostasis diagnostics, driving technological innovation to deliver advanced solutions for global healthcare development.